HOME > INTERNATIONAL CONFERENCE ON MEDICAL DEVICE SAFETY RISK MANAGEMENT
ICMDRM 2025
Medical Devices Mustn’t Fail Your Business—or Your Patients

Find Francois Retief in the St. Cecilia Chamber to discuss systems engineering training, spin the wheel to win a prize and grab a chocolate!
All trademarks, logos and brand names are the property of their respective owners.
Restrictions apply, see amazon.com/gc-legal

Project Performance International (PPI) is proud to sponsor this conference! Meet Francois Retief in the St. Cecilia Chamber to explore how PPI’s systems engineering training and consulting can help your business take a structured approach to ensuring medical products meet safety, performance, and regulatory standards—while reducing failures, delays, and costly rework.
PPI is a global leader in systems engineering (SE) training and consulting, helping engineers design safer, more reliable, and regulatory-compliant medical devices. Our training equips professionals with the skills to identify and mitigate risks, streamline development, and enhance product performance, ensuring compliance with ISO 14971 and other industry standards.

Reduce risk & improve compliance
Apply structured SE approaches to meet regulatory requirements.
Ensure safety & reliability
Own risk management to prevent failures, delays and disastrous product recalls.

Enhance
efficiency
Optimise processes to accelerate development without sacrificing quality.
Round Table Discussion
Title: Balancing Innovation, Speed, and Safety in Healthcare: The Role of Systems Engineering and Systems Thinking
Location: King Room
When: 25 April 15:30-16:15pm
Overview:
The healthcare industry faces mounting pressure to innovate rapidly while reducing costs and improving efficiency. However, accelerating development cycles and streamlining operations must not come at the expense of patient safety and risk management. Systems engineering and systems thinking offer structured approaches to considering all relevant needs, balancing competing demands for the most beneficial outcome, and ensuring that innovation remains both sustainable and safe.
This interactive roundtable will provide a platform for participants to engage in a dynamic discussion on the kind of problems typically encountered and how these methodologies can enhance decision-making, improve process integration, and optimise risk management in the face of industry pressures. Guided by a moderator, attendees will explore key topics such as designing resilient yet efficient healthcare systems, integrating human factors into rapid innovation, and leveraging cross-disciplinary collaboration to maintain high safety standards without compromising speed.
Participants will have the opportunity to share experiences, ask questions, and debate best practices in an increasingly fast-paced medical landscape. Join us for an engaging discussion on practical strategies to drive innovation while safeguarding patient outcomes and regulatory compliance.
Let's connect
Looking to strengthen your team’s approach to medical device risk management? Let’s connect to discuss how PPI’s systems engineering training and consulting can help you enhance safety, ensure compliance, and reduce risk in medical product development.

Francois Retief
PPI Course Presenter and Principal Consultant
About PPI
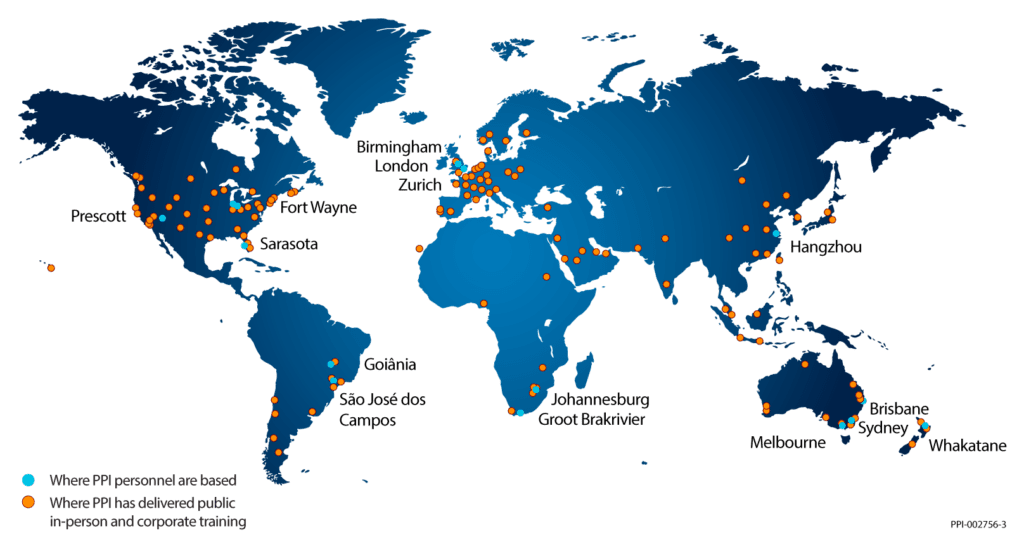
With a track record of training 20,000 professionals across six continents and 42 countries, PPI is a global leader in systems engineering training and consulting. Whether you are an industry veteran, a novice, or a manager seeking to improve your team’s performance, PPI’s training delivers results that make a lasting impact.
“The content delivered was of the highest standard, and I am truly appreciative of the time and effort invested by the instructor. The materials shared during the course have been instrumental in expanding my understanding and equipping me with practical insights that I can directly apply to my work starting today.”
— Course Participant, United Kingdom
PPI training courses
PPI offers a range of comprehensive training courses to meet the needs of systems engineering professionals at every level. Our courses provide practical knowledge and skills that can be immediately applied to real-world projects.
Our Most Popular Courses:
- Systems Engineering 5-Day Course: Master the principles and best practices to reduce time and cost, delight stakeholders, and minimise errors in technical projects. (PPI’s flagship course)
- Requirements Analysis & Specification Writing 5-Day Course: Accelerate project delivery by learning how to write and validate superior requirements that ensure defect-free, on-budget outcomes.
- Systems Engineering Management 5-Day Course: Gain the skills and confidence needed to lead and manage complex engineering projects effectively and efficiently.
- Architectural Design 5-Day Course: Improve project outcomes by employing sound design principles and efficient design methods.
Explore all of our training offerings and find the right course for you and your team.
Capitalise on our free resources
At PPI, we believe in empowering the engineering community with knowledge. Explore our extensive collection of free resources designed to keep you at the forefront of systems engineering:
- Articles & Presentations: Dive into our curated collection of insightful articles and presentations that keep you ahead in systems engineering.
- Systems Engineering Key Downloads: Access a wealth of downloadable presentations, short papers, specifications, and more—tools designed to enhance your projects.
- Systems Engineering Goldmine: Explore over 5GB of valuable information and a searchable database of 8,500+ terms to support your work.
- PPI-INCOSE Systems Engineering Training Database (SETDB): A comprehensive database that helps you to find appropriate software tools and cloud services that support your systems engineering activities.
- PPI Systems Engineering Newsjournal (PPI SyEN): Stay updated with in-depth professional articles, briefings, and the latest systems engineering news each month.
We look forward to seeing you at the International Conference on Medical Device Safety Risk Management and connecting with you in one way or another!
Have a question?
Get in touch to discuss ICMDRM 2025, systems engineering training, or PPI’s FREE resources!
All trademarks, logos and brand names are the property of their respective owners.
